Journal club - Permutations of Immunoblotting
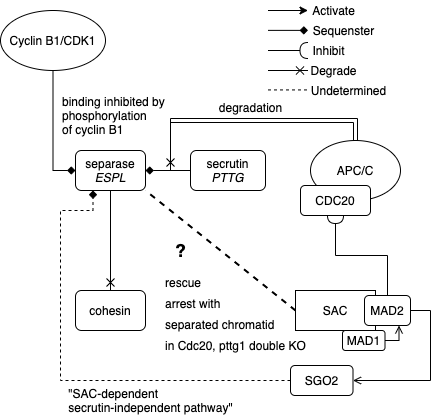
Sister chromatid separation is triggered by cohesin cleavage mediated by separase. Therefore, it is important to keep separase in check. There are two previously defined pathways that lock separase up.
- Securin complexes with separase until until being degraded at late metaphase by APC/C. Inhibition of CDC20 on APC/C is dismantled by SAC when the spindle attaches to all the kinetochores.
- Cyclin B1-CDK1 inhibits separase before it is degraded by APC/C during metaphase. Early phosphorylation of cyclin B “dampens” its inhibitory function.
When Cdc20 and Pttg (securin) are both KO, chromatid separation defects are rescued by constitutive SAC activation. So, there can be securin-independent separase release/activation. Hellmuth and colleagues (2020) delineate a novel pathway in which separase is seized by SGO2 and MAD2, aside of the canonical securin-separase complex, and released by TRIP13 & p31 signalling, possibly by APC/C-dependent ubiquitin proteasome system (UPS) as well. The intricate regulation network is summarized as below.
Can SGO2, MAD2 complex with separase?
SGO2 $\rightarrow$ separase, MAD2 $\rightarrow$ separase, MAD2 $\rightarrow$ SGO2 (substrate $\rightarrow$ antibody) are confirmed by Co-IP (1a) and “consecutive immunodepletion” (1b).
Consecutive immunodepletion: Protein mixture before and after antibody-wash (“immunodepletion”) are subjected to immunoblotting. In 1b, SGO2 antibody can pull separase, MAD2 down from the protein mixture. After treated with SGO2 antibody, separase antibody can no longer pull separase, MAD2 or even SGO2 itself down, proving tight bounding between the three elements.
SGO2 mutants were then exogenously expressed while endogenous SGO2 are KD by siRNA. Lysates from these cells were subjected to myc-IP. In 1c, Myc-tagged separase were pulled down together with SGO2(WT/R58I) and MAD2 but not SGO2(R153D). R153D is an MAD2-binding defect and R58I the PP2A-binding defect, confirming SGO2 binding to separase is MAD2-dependent. The functional readout of SGO2 is the percentage of premature chromatid separation (PCS). Still, SGO2(WT/R58I) but not SGO2(R153D) can rescue increased PCS phenotype when endogenous SGO2 is depleted.
Now that separase-SGO2-MAD2 interaction is established, its dynamics as for cell cycle phases are tested. 1d showed ubiquitous existence of the complex over 4 cell cycle phases. 1e showed time coupling of progressive SGO2/MAD2 loss with separase cleavage after cells were synchronized and bypassed SAC using taxol-ZM treatment.
Taxol - ZM-447439 treatment Taxol is a microtubule stabilizer which can synchronize cells at mitosis. ZM-447439 is an aurora B kinase inhibitor that silences SAC and drives cells into “anaphase-like state”.
Because cell cycle distribution of SGO2-MAD2 in 1d is similar to CDC20-MAD2 which require MAD1/TPR activation, researchers tested SGO2 response to KD MAD1 or TPR. In 1f, SGO2 antibody cannot pull MAD2 or separase (almost) when either MAD1 or TPR is KD; CDC20 antibody cannot pull MAD2 down when KD (previously known, for validation).
Is SGO2 functionally significant?
In somatic cells, both securin and SGO2 are not essential. However, co-depletion using RNAi destroyed clonogenic capacity of the cells (2a). Akin to this functional readout, PCS level after taxol-synchronization greatly increased if securin-SGO2 or securin-MAD2 were co-depleted (2b).
On the molecular basis, RAD21, a subunit of cohesin, were cleaved only when securin and SGO2 were both depleted after taxol-synchronization (2c). Moreover, in the context of securin-separase co-depletion, only WT exogenous separase could be captured by SGO2, not V4/V5 mutant that could not interact with SGO2-MAD2 (2d).
Overall, the seizing role of SGO2-MAD2 was validated at both phenotypical and molecular levels.
How separase is bound with SGO2-MAD2?
Securin inhibits separase by occupying its catalytic site. As securin cannot be cleaved, the separase is inactivated. Researchers tested whether SGO2-MAD2 inhibits separase in the same manner. RAD21 cleavage was adopted as the function readout of separase activity. From now on, experiments were carried out in vitro at first and then in vivo.
To validate in vitro assay, researchers first replicated SGO2-MAD2 inhibition effects on separase. Combinations of SGO2 and MAD2 variants were used. Only when MAD2 C-terminal is intact and SGO2 interaction with MAD2 is not damaged can separase be prevented from cleaving RAD21.
| Variant | Feature | Outcome |
|---|---|---|
| SGO2R153D | MAD2-interacting defect | RAD21 cleaved in vitro no matter MAD2 type |
| SGO2N58I | PP2A-interacting defect | RAD21 not cleaved in vitro with MAD2 WT |
| MAD2$\Delta C$ | No C-terminal | RAD21 cleaved in vitro no matter SGO2 type |
To test whether SGO2 inhibits separase in a manner similar to securin in vitro, they mutated putative SGO2 pesodusubstrate site into real substrate site. In 2b, PD separase could never cleave SGO2; SGO2 variant M114R (not F95R, S126R) can be cleaved by active separase, the amount of cleavage is increased by Polo-kinase.
In taxol-arrested cells (in vivo) with both WT SGO2 and securin depleted, they tested either replacing SGO2 with identified M114R or M114R+239-242A mutant (compromise interaction with separase), or replacing secruin with F118R mutant (entitle secruin to be cleaved by separase). Variants are FLAG-taged.
Co-IP using FLAG antibody in secruin-depleted cells showed that separase could cleave M114R SGO2 at N terminal but not WT or M114R+239-242A SGO2 (3c lane 1~3). Co-IP using SGO2 antibody in SGO2-depleted cells showed that F118R secruin could also be cleaved at the C terminal but not WT, which serves as a positive control (3c, lane 4~5).
Indeed, secruin is an competitive inhibitor of separase and the inhibition is MAD2-dependent.
How separase is released from SGO2-MAD2?
It is accepted that CDC20-MAD2 complex (MCC complex) is dismantled by TRIP13 and p31, allowing for SAC-MAD2-CDC20 -APC/C-UPS signalling. Researchers hypothesized that TRIP13/p31 are involved in the cleavage of SGO2-MAD2 from separase as well. In vitro, separase-precipitated complex is immobilized and treated with TRIP13/p31. The supernatant was taken for detecting released SGO2/MAD2 while the remaining was washed, tested for separase activity via substrate RAD21, then subjected to constituent analysis (4a). The presence of SGO2 and MAD2 in the supernatant, RAD21 cleavage and little remaining SGO2 or MAD2 on beads all confirmed in vitro release of separase mediated by TRIP13 and p31. In vivo, $\Delta_{\text{RNAi}}$ securin taxol-ZM treated “anaphase” cell were compared with $\Delta_{\text{RNAi}}$ securin $\Delta_{\text{RNAi}}$ TRIP13 $\Delta_{\text{RNAi}}$ p31 ones. Separase-SGO-MAD2 complex are maintained (4b).
| Variant | Feature | Outcome |
|---|---|---|
| TRIP13Walker A/B | Inactive | No SGO2/MAD2 cleaved in vitro |
| p31QF | MAD2-binding defect | Little SGO2/MAD2 cleaved in vitro |
| p31PK | TRIP13-binding defect | No SGO2/MAD2 cleaved in vitro |
If TRIP13/p31 works, can separase get released independent of APC/C?
Researchers treated taxol-ZM cells with proTame and Apcin - both are APC/C inhibitors. Within 35 minutes after release, chromatid separation appeared more quickly in PTTG-/- cells in which separase should be predominantly sequestered by SGO2-MAD2 instead of securin (4c). Separase-IP showed continuous SGO2 and MAD2 decrease upon release whether there is securin or not (4d), confirming the notion that when APC/C is silenced, SGO2-MAD2 takes the main control and allows chromatid separation.
Comments
The paper introduced the novel pathway with a concrete logic from biochemistry interaction, functional outcome to signalling regulation. The Western blot permutation on variants were carried out through replacing endogenous genes (RNAi) with exogenous versions. This trick is less labor insensitive than knock-in, nevertheless, short functioning time and less predictive effects (e.g. overexpression of Figure 2c probably amplified by basal, endogenous protein) makes it unsuitable for long-time assay. Joint efforts from those two “modes” of experiments can be made.
Reference
Paper discussed
HELLMUTH, S., GÓMEZ-H, L., PENDÁS, A. M. & STEMMANN, O. (2020) Securin-independent regulation of separase by checkpoint-induced shugoshin–MAD2. Nature, 580(7804), 536–541.