Journal club - 2D or 3D culture?
Chromosome missegregation and the consequent aneuploidy are lethal to normal cells, though, fitness of cancer cells is paradoxically enhanced by aneuploidy.
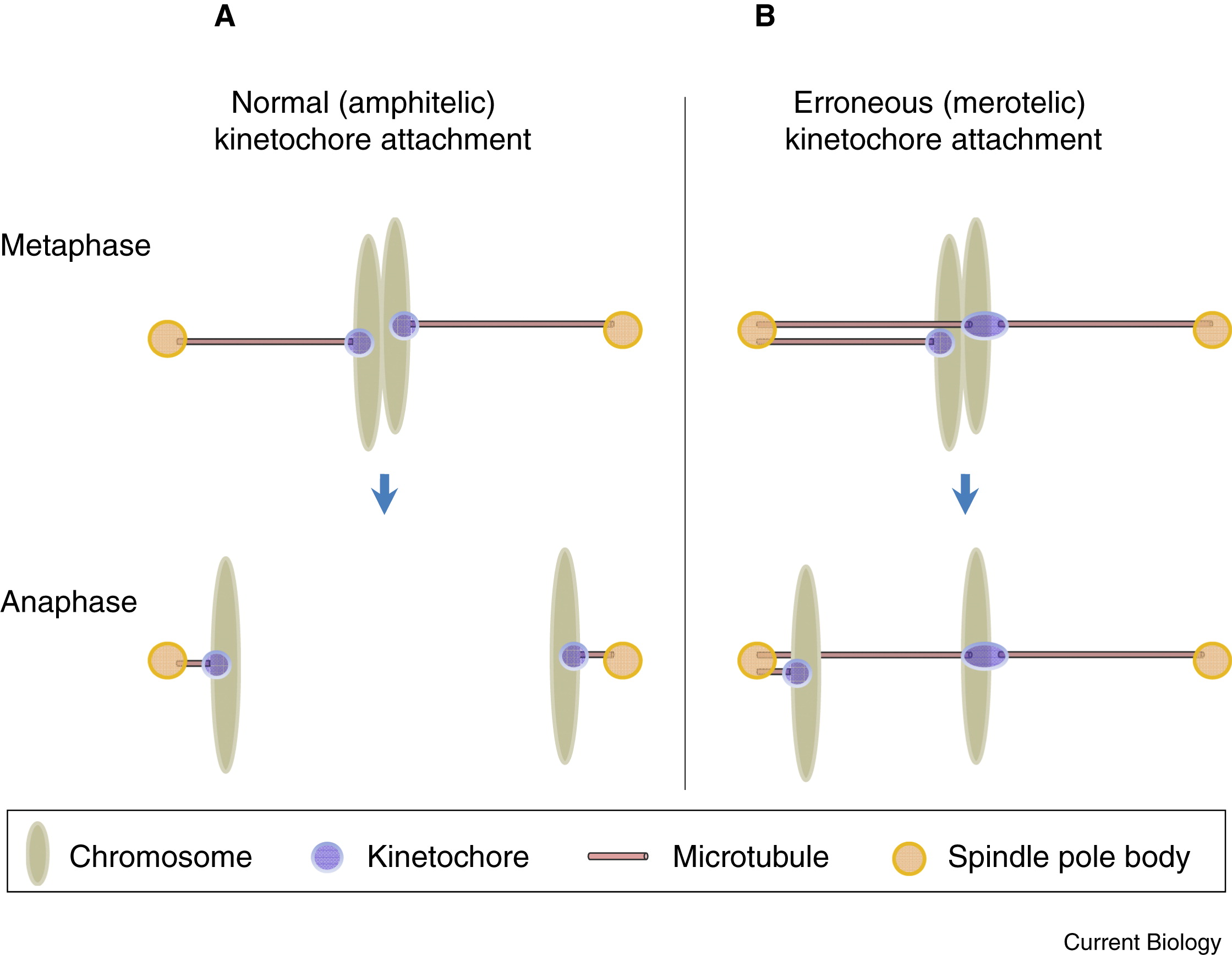
Chromosome segregation is executed and regulated precisely. When a pair of sister chromatids segregates, microtubules of the mitotic spindle attach to the kinetochores and drive the chromatid towards the direction of spindle pole. We can imagine that multiple errors could happen during microtubule attachment.
- Microtubules from the same spindle pole as sister kinetochore attached to the kinetochore (wrong-to-1).
- Microtubules from neither spindle poles attached to one kinetochore (0-to-1).
- Microtubules from both spindle poles attached to one kinetochore (2-to-1).
Abnormal attachment failed to generate tension for separation, which is eliminated by the spindle assembly checkpoint (SAC). As for the third case above, called merotelic attachment, chromatid may stretch in an extent to escape SAC. This chromatid lags behind the separated ones during anaphase. Therefore, it may be incorporated into the wrong daughter cell. No matter its cell affiliation, it is possible to be excluded from the nucleus, thus forming micronucleus in which chromosome is exposed to DNA damage during cytokinesis. In many cancer cell lines, ~50% cells possess micronuclei or lagging chromosomes (Cimini et al., 2001; Thompson and Compton, 2008, 2011).
On top of the cellular mediators of chromosome segregation, environmental factors can influence the process given the following evidence
- Adhesion patterns to ECM is transduced through integrins into the cell (Lechler and Fuchs, 2005; Thery et al., 2005).
- Mechanical manipulation influence cell rounding during mitosis (Itabashi et al., 2012; Lancaster et al., 2013).
It’s even more interesting to know that normal hepatocytes harbor supernumerary centrosome but keep polyploidy in liver tissues whereas cultured hepatocytes develop chromosome missegragation and aneuploidy (Duncan et al., 2010, 2012; Knouse et al., 2014). To plus, unexplained chromosome instability of cultured cancer cells with functional SAC may be attributed to the ex-vivo environment (Haruki et al., 2001; Tighe et al., 2001).
By comparing the chromosome segregation fidelity of various cell types in cell culture with those in native tissues, Knouse et al., 2018 showed epithelial cells loss some chromosome segregation fidelity in 2D culture, which is attributed to integrin function. By using 3D organoid culture, they showed how tissue architecture rescues merotelic attachments in hepatocytes.
Note: Introduction above is paraphrase-adapted from Knouse et al., 2018. References are taken from the manuscript.
Gross relationship between chromosome segregation fidelity and tissue architecture
It is assumed that cells loss their tissue architecture after dissociated from the native tissue. Researchers separated cells from mammary gland, skin, neonatal liver, embryonic brain and lymph node. The readout of chromosome segregation error i.e., lagging chromosomes and micronuclei are measured through staining. Figure 1b showed significant increase of these two indices in more structured mammary gland, skin and neonatal liver. Epithelial cells in the embryonic brain and lymph node do not show such difference.
3D culture (Matrigel) is shown to rescue tissue architecture. In 1d and 1e, epithelial markers ($\alpha6$ Integrin, ZO-1: tight junction component) gradually emerge from 48 hr (“immature”) to 96 hr (“mature”) Matrigel culture, in parallel with reduction in lagging chromosomes and micronuclei. In this vein, a relationship between chromosome segregation fidelity and tissue architecture is established.
Shape and polarity: direct readout of tissue architecture
Cell shape and polarity directly reflects tissue architecture. In a 3D coordinate, researchers plotted length of 3 orthogonal directions of single cells. By comparing dissociated, native cells or Matrigel cells at interphase or mitosis, unique patterns emerge.
- Native/Matrigel cells do not round up during mitosis as in dissociated 2D culture (2a, 2c).
- During mitosis, mature Matrigel cells have more similar morphology (shape and polarity) to native cells; dissociated cells are flattened (2b).
- Immature Matrigel cells are different from mature ones, but the difference is trivial regarding difference between dissociated and native cells (2b, 2c).
Mediator identified: integrin
In Cre-ERT2, a drug-inducible KO system, researchers depleted integrin which is a protein that connect cytoskeleton to extracellular matrix. Cell shape has slightly changed (2e). The important finding is that lagging chromosomes and micronuclei increase greatly after integrin KO. Therefore, researchers concluded that integrin mediates cell adhesion to ECM and thus contribute to chromosome segregation fidelity.
How to enhance fidelity: Correction of merotelic attachments
Now that immature and mature Matrigel cells resemble poor and good tissue architecture respectively, researchers set to determine how tissue architecture rescue chromosome segregation errors observed in 2D culture.
Tissue architecture may promotes chromosome segregation fidelity in four ways,
- Regulate segregation-related gene expression;
- Regulate SAC function;
- Reduce merotelic attachment formation at metaphase;
- Promote error correction of merotelic attachment in late mitosis.
RNA-seq data in 3a displayed parallel increase of proliferation-related and segregation-related gene expression during Matrigel cell maturation, excluding the first possibility.
The rest three possibilities are tested by evaluating mitosis dynamics because merotelic attachment is responded by SAC and is rendered into transition time among mitotic phases. In 3b, prometaphase to anaphase transition time is reduced in mature Matrigel cells compared with immature ones. The difference is eliminated by reversine-induced SAC silencing. To dissect this timing further, time course of chromosome segregation is divided into 2 parts: prometaphase to bipolar (centrosome movement) and bipolar to anaphase (chromosome movement). The major time difference in 3b is contributed by chromosome movement timing, which is a clear evidence of SAC signalling. Hence, SAC is functional in both conditions, akin to many cancer cell lines, excluding the second possibility.
Next, researchers asked whether prolonged SAC signalling is activated by an increase of merotelic attachment. Since merotelic attachment may be caused by supernumerary centrosome or delayed centrosome separation. The second situation is measured as chromosome congression delay. However, neither of them can be detected (3d), excluding the third possibility.
Finally, researchers turned to the correction pathway that spare erroneous merotelic attachment for future attachment.
In 3f, 3/26 Matrigel cells showed chromosome expelled from the metaphase plate, which does not happen in 30 mature cells. This unique chromosome movement can be explained by sudden loss of the microtubule attachment. Combined with prolonged SAC signalling time, they concluded that proper attachment cannot be established after the error one is cleaved. Because they did not observe morphological changes of the spindle, they suspected that the loss of tissue architecture may influence tension of the spindle, therefore make it more difficult for correct attachment to form.
Application of our knowledge to polyploid hepatocytes
There is one natural situation when chromosome segregation fidelity should be deliberately maintained - polyploid hepatocytes. Hepatocytes can maintain their genomic stability even with multiple spindle poles during mitosis. Moreover, hepatocytes have distinct regenerative capacity, therefore suitable for an in-vivo VS ex-vivo assay. Regenerating hepatocytes harbor much less lagging chromosomes and micronuclei than dissociated hepatocytes (4b). Herein, the paradoxical polyploidy and mitotic activity converges.
Comments
The paper showed an important role of tissue architecture related to chromosome stability, which is a very natural idea but directly hit the major limitation of 2D cell culture. In the mainstream of cancer cell biology, it may be worth considering to validate conclusions related with genomic stability, stress response and structure dynamics in 3D culture.
One issue of using primary cells for comparison is that defects caused from tissue dissection cannot be eliminated. A rescue trial using well-adapted, cultured cells seeded into organoid is suggested.
Reference
Paper discussed
KNOUSE, K. A., LOPEZ, K. E., BACHOFNER, M. & AMON, A. (2018) Chromosome Segregation Fidelity in Epithelia Requires Tissue Architecture. Cell, 175(1), 200-211.e13.